The brain is the most complex organ in our body. It is also the most difficult to reach. These twin complications drive the massive attrition of new brain therapies, 85% of which fail in phase II or III. Successful therapies must either be safe and effective when delivered systemically or be able to navigate the brain precisely to where they’re needed. Therapies suitable for widespread use must also be able to deliver themselves to the brain without damaging and invasive neurosurgery which would discourage patients from using them early, instead of only when they have no other choice.
We’re creating a new class of cell therapies for the brain inspired by nature capable of solving both these problems. Our cell population delivers itself to the brain after intravenous infusion, and homes only to signs of damage and disease when it arrives there. It then integrates into these damaged sites, differentiating into many different types of functional, adult brain cells and repairing the underlying damage by replacing lost cells with healthy ones. Interested in learning more?
Your Brain on Pregnancy
Pregnancy is a well of biological mysteries that is still far from exhausted. Previously, I wrote about a rare population of fetal cells able to cross into the mother’s body during pregnancy, where they home to her heart and can even repair severe damage from heart attacks. It turns out a similar population of fetal cells can make it into the mother’s brain, and their healing potential is no less powerful. No one has used this population as an allogeneic therapy outside the mother similarly to what was done with the heart targeting feto-maternal subpopulation I covered in the article linked above, but its therapeutic potential has caught the eye of several researchers working with pregnant mice.
Starting with this 2005 study in Gavin Dawe’s lab at the National University Of Singapore, researchers crossed male mice engineered to express Green Fluorescent Protein (GFP) throughout their bodies with unengineered females, resulting in GFP expressing offspring who’s cells could easily be tracked within the mother’s body during pregnancy and after birth. Various names exist for this cell population, but I’ll go with the one I used in my previous essay: feto-maternal microchimeric stem cells or FMSCs. They found a very small population of FMSCs (about 7 for every thousand of the mother’s cells on the day of birth and 5 per thousand four weeks after) made it into the brain as a result of pregnancy, and that this population increased six-fold when they chemically injured the mother’s brains. This increase was only found in the injured tissue, showing that these cells could cross the blood-brain-barrier and home to injuries within the brain.
Dr. Dawe’s group followed these brain-homing FMSCs over time, removing brain tissue at the day of birth and 4 weeks postpartum and characterizing which surface proteins the green population expressed with immunostaining, while also visually observing their changes in shape and behavior with confocal microscopy. These cells split into two distinct populations in the injured models. Some hugged the walls of nearby blood vessels and expressed mouse macrophage marker F4/80 and took on elongated, macrophage-like morphologies, while others clustered around the chemical lesions and expressed markers associated with neuron, astrocyte, and oligodendrocyte lineages. And it wasn’t only their surface proteins which changed when they integrated around injured sites—these new brain cells looked the part, too.
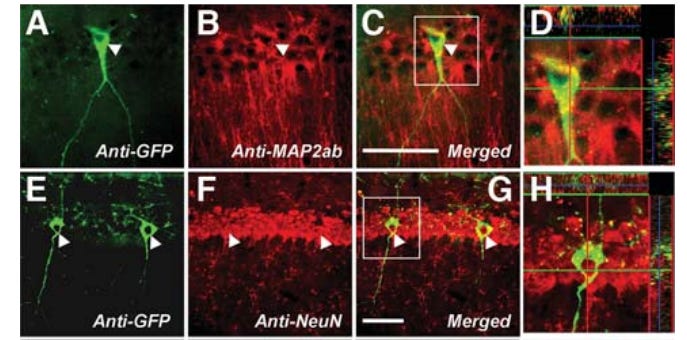
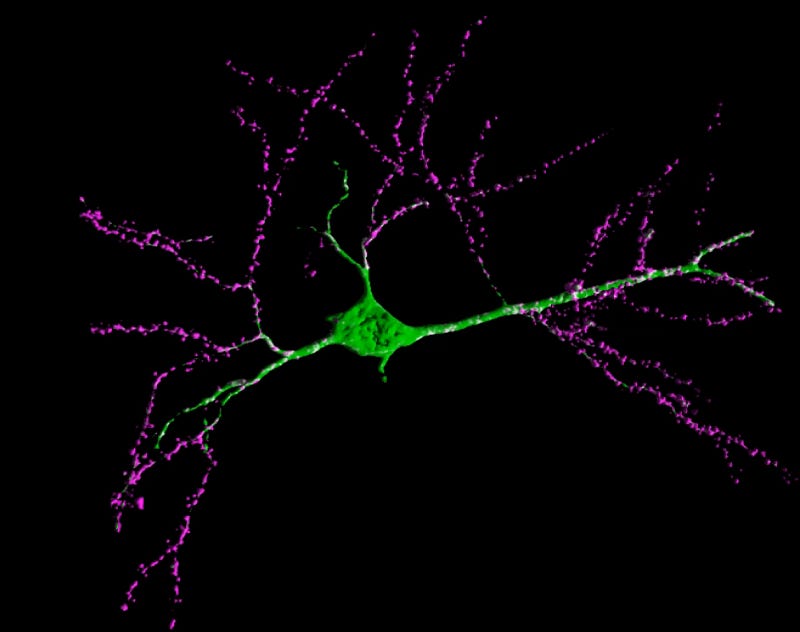
Dawe’s group expanded on this study in a follow-on paper, investigating a few key facts for the potential translation of this cell population into a therapeutic for indications in the human brain. While their first study only checked for FMSCs in the maternal brain four weeks after pregnancy, this one still found them seven months after birth. Instead of only observing the fetal cells expressing neuron-like markers and morphology at their earlier timepoint, the authors followed their differentiation from multipotent stem cells to adult neurons, observing the new cells in the mother’s brain expressing fully mature neuronal surface markers and morphology 60 days after birth. This mature cell population was also mostly composed of neurons, with no glial markers observed in the day 60 population. These results were obtained from healthy brains lacking the lesions induced in the previous study, showing that brain trafficking FMSC populations might skew towards becoming neurons during long term engraftment in healthy recipients, while differentiating into a broader range of brain cells when targeted towards damaged (lesioned) tissue. However, they only conducted this differentiation analysis on FMSCs extracted from the maternal hippocampus, possibly missing non-neuronal differentiation in other brain regions.
This study took one more important step past the 2005 paper: studying FMSC behavior in a specific disease model. Here, they selectively killed dopaminergic neurons in the right striatum of some of the mouse mothers’ hippocampi, inducing symptoms similar to Parkinson’s disease. FMSCs were twice as likely to home to this damaged tissue in the first 60 days after birth, but their population in the damaged striatum collapsed after that point with 95% of FMSCs in the injured brains found in other areas of the hippocampus post 60 days. This suggests FMSCs will home to injured tissue in more complex diseases such as Parkinson’s, but might require maintenance through repeated therapeutic delivery in order to help regenerate damage from the disease.
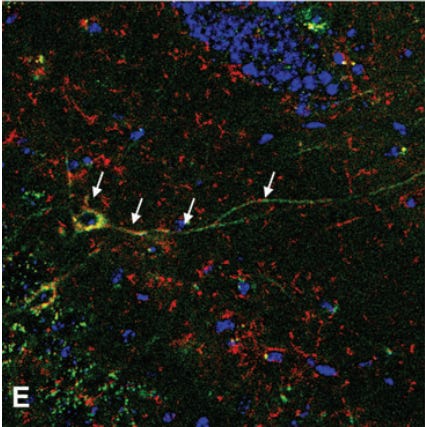
These studies from Gavin Dawe’s lab did much to characterize this rare population of brain targeting fetal cells and describe how they behaved in injured vs uninjured models, but didn’t make any attempts to use them as a therapy. I’m not yet aware of any lab which has purified the brain targeting FMSC population and used it as an allogeneic treatment against a set indication as described in the Chaudry lab’s earlier works, but Dr. Sélim Aractingi’s lab at the Université Paris Cité came closest to that in 2022 with this paper. Here, they injected CCL2 (a chemokine known to attract brain trafficking fetal origin cells) directly into the brains of wild-type female mice which had previously given birth to three GFP+ litters. This treatment increased FMSC concentration in the brain by 10x compared to untreated controls, serving as a proxy for the possible effects of injecting high concentrations of brain trafficking FMSCs directly. Similar to earlier studies in the Dawe lab, the authors chemically induced ischemic stroke-like lesions in the brains of some of their mice. Injecting lesioned brains with CCL2 caused more FMSCs to integrate into the lesions, shrinking their size by half five days after treatment.
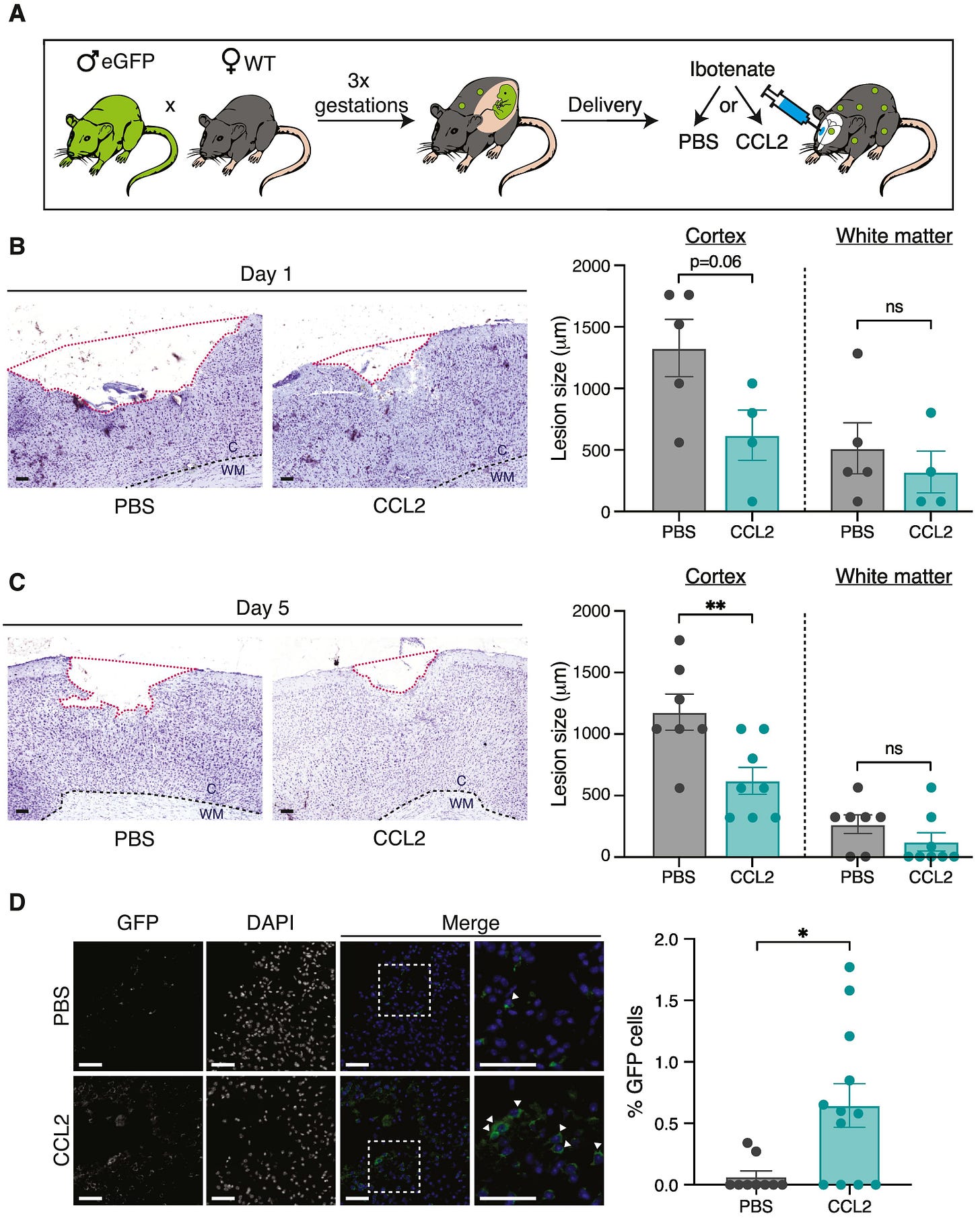
This functional outcome was deeply inspiring, especially when it was achieved with a single treatment and only by recruiting the existing brain-targeting FMSCs circulating in the animal’s systems instead of introducing new ones. It immediately got me thinking about what we could achieve with higher, repeated doses exclusively comprised of this brain targeting sub population—not only in stroke and Parkinson’s, but across all neurodegenerative diseases. However, the part of this paper which convinced me to start a company to pursue it was more subtle.
CCL2 isn’t an isolated signaling molecule only found in this pathway. Its participation in a range of physiological processes is very well characterized, most importantly, in aging. Aged cells upregulate CCL2 excretion across the body, and the chemokine is recognized as a key component of the Senescence Associated Secretory Phenotype (SASP), a hallmark of aged and nonfunctional cells. If we can find a therapeutic cell population which delivers itself to the brain and then seeks out areas of high CCL2 expression within it, we will have closed a critical step to being able to replace aged brain tissue with young, healthy cells without the need for surgery.
These papers are inspiring, but raise several questions we’ll need to answer while working to harness their insights as a therapy.
First, none of the papers actually gathered behavioral observations of the treated mice to prove that FMSC integration into lesion sites led to observable recovery from stroke. Aractingi’s group cited previous studies showing that mice who had experienced multiple pregnancies before a stroke recovered better than those which hadn’t and stated that their results were “in agreement” with those studies, but no explicit data was shown. We’re very excited about the potential of FMSC based brain therapies based on the cellular data, and have plans to conduct extensive behavioral studies ourselves to test if they can actually improve cognition and performance after stroke.
Another question raised by Aractingi’s paper revolves around the size of brain lesions in multiparous vs virgin females. CCL2 treatment did reduce lesion size by about 50% in multiparous females, but the untreated multiparous females had lesions more than twice the size of their virgin counterparts. This means that multiparous females were still left with larger lesions than mice which had never been pregnant even after CCL2 driven FMSC integration into their brains. This leads me to believe that something about the state of repeated pregnancy led to larger lesions in these stroke models (a factor avoided when directly delivering these cells as a therapy), but the observation warrants further investigation.
Finally, Dawe’s papers suggested a pronounced skew in FMSC differentiation depending on the brain microenvironment they implanted in. Cells exposed to fresh lesions were skewed towards becoming a range of glial cells, while those implanting into healthy brains differentiated entirely into neurons. Investigating the effect of this skew on recovery when treating a range of neurological indications will need to be one of our top priorities, as well as engineering ways to tilt it towards therapeutically favorable distributions.
Baby Cells, Mature Market
Neurological degeneration driven by aging or disease is one of the most complex ways in which the human body fails, and it happens in our most complex and isolated organ. I believe self delivering cell therapies capable of sensing and adapting to the changing brain environment will be the key to solving these thorny problems—but developing them to that point will require obscene amounts of money. We’ll need to turn a significant profit quickly if we hope to advance this therapy ourselves, and I see two routes for doing so:
Pharma Partnerships:
Big pharma voraciously searching for new ways to get their drugs past the blood-brain-barrier. From GSK’s recent $2.5B ticket acquisition of ABL Bio’s antibody-based BBB shuttle to AbbVie’s $1.4B outright purchase of Aliada Therapeutics for their BBB-crossing antibody therapeutic against Alzheimer’s, it’s clear that this market is picking up speed. However, the most mature shuttles are currently viral capsid or antibody based, delivering their cargo past the blood-brain-barrier systemically and at low efficiency. This is a massive limitation for therapies requiring higher concentrations for their full effects or which have the potential to be toxic at high doses (anti-Aβ antibodies such as Aliada’s have shown this in the past), and viral delivery or non-humanized antibodies also run the risk of triggering an immune dangerous immune response in the brain.
A cell-based BBB shuttle mitigates all these concerns, especially one capable of seeking out and integrating into sites of damage. The most valuable emerging neurological therapies aren’t small molecule drugs, but proteins with neuroprotective effects. Using our cells to deliver therapeutic proteins or the mRNA which codes for them will greatly improve current methods which can only deliver systemically and at low doses, allowing us to target protein-based medicines precisely to the sites which need them. This also transforms our therapies into something more than simple symptom mitigation—rebuilding the patient’s brain to produce its own medicine over time or upon exposure to a small molecule or other cue and permanently changing their life for the better. Our FMSC based tech has the potential to moonlight as a best-in-class BBB shuttle, opening partnership avenues to fuel its development into a fully fledged therapy. Such as:
First Indication
Sundial has decided to pursue ischemic stroke as our first indication for the following reasons:
Existing data: Most of the current literature on these cells evaluates them in systems modeling this condition.
Easy to test: Brain lesions are relatively easy to chemically induce and better model actual stroke, as opposed to more controversial systems such as high Aβ mouse models for Alzheimer’s. Behavioral impairments caused by stroke are also easy to observe, as are the cell-level interactions between our therapy and the injury site (lesion shrinking as observed in the papers above). Straightforward assays and clearly deliverable evidence of efficacy have the potential to greatly decrease the cost of bringing this therapy through the FDA, bringing it within the reach of a startup supplemented by partnership money without requiring the kind of war chest only a big pharma acquisition could deliver.
Valuable market: This therapy would be the first to actually cure the underlying damage caused by stroke and restore lost cognitive abilities, potentially unlocking a massive market to further fuel the development of these therapies.
Estimated Revenue:
Time for some back of the envelope math. Allogeneic stem cell therapies are a very new market, and none of yet been approved for use against neurological indications. This makes estimating the potential cost of our therapy a tricky proposition, best achieved by pegging it against:
Existing allogeneic stem cell therapies: While many of these therapies are working their way through the FDA, none are yet for sale in the U.S and vanishingly few are available anywhere else in the world. Our best comparison is TEMCELL, an allogeneic mesenchymal stem cell therapy product currently selling for between $113 and $170K per course of treatment in Japan. It’s also an IV delivered, multi-treatment allogeneic therapy, making it the best current comparison to our product despite not treating a neurological indication.
Economic burden of the condition: The lifetime cost of ischemic stroke has been estimated at $140,048 to the patient by the American Stroke Association, and $59,900 per year in the U.S by this review paper.
Putting these figures together, it’s not unreasonable to expect that payers would be willing to pay around $150K for a curative ischemic stroke therapy which prevents that $140K cost while also restoring cognitive function to the patient, a number falling within TEMCELL’s current range. That’s the per-treatment price I’ll use when moving forward to estimate potential revenue.
Total Addressable Patient Base: 7.8 million ischemic stroke cases occur each year around the globe, but our therapy wouldn’t be able to treat them all. Some strokes are ‘silent’, meaning they don’t manifest symptoms which would drive the patent to seek treatment, while others are immediately lethal. Hospital access remains the tightest limit on getting these therapies to patients, however. Ischemic strokes are caused by blood clots cutting off oxygen supply to the brain, and current treatment practices involve clearing these clots within 4 to 24 hours of the stroke to minimize brain damage. We can use ability to receive these interventions within the recommended time window as a proxy for for access to advanced hospital facilities which also more or less filters out asymptomatic and lethal strokes. This 2021 review paper claimed that less than 5% of global stroke patients receive pharmacological clot clearing agents within their critical window each year, while fewer than 100K have their clots cleared mechanically. Although these numbers are drawn from all global stroke incidents (only 87% were ischemic), I’ll round up to 6.28% of global patients falling in our treatability window (5% + that 100K (1.28% of global incidence)). As mentioned above, our therapy would be an IV infusion deliverable in much lower-tech facilities with less trained staff than those required for thrombectomies (clot-clearing), which means that even rounding up here leaves us with an aggressively conservatives estimate of the number of patients we can treat each year: 6.28% of 7.8 million—about 490,000. We wouldn’t capture this market right away either. But as a fist-in-class therapy, we could grow into it.
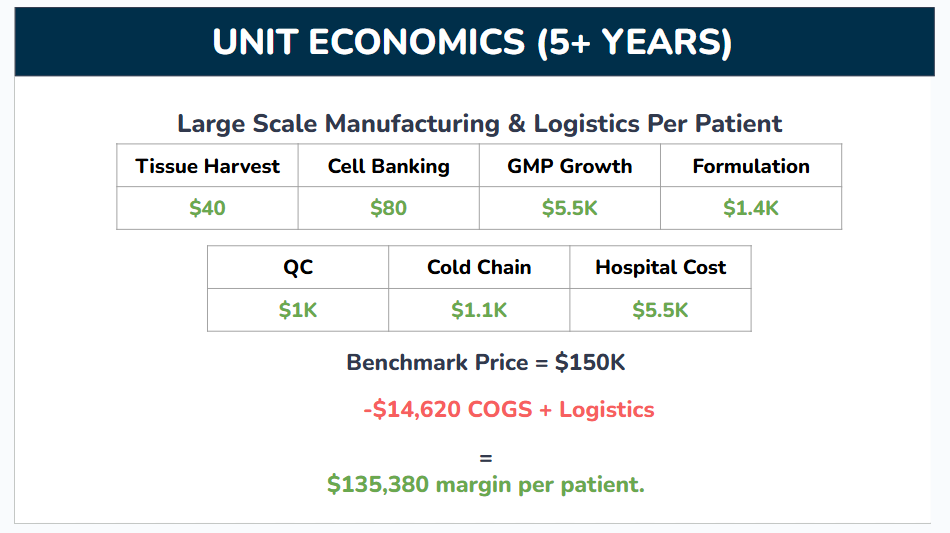
Unit Economics
Now that we have an estimate of our potential annual patients, let’s dig into how much these therapies actually cost to create. Even an extremely expensive therapy capable of actually regenerating damage to the brain would be better than the current market alternative—nothing. But it falls far short our vision to make brain regeneration widely available and build the foundations of an accessible treatment for age related degeneration in the brain. Luckily, the numbers stack up in our favor again:
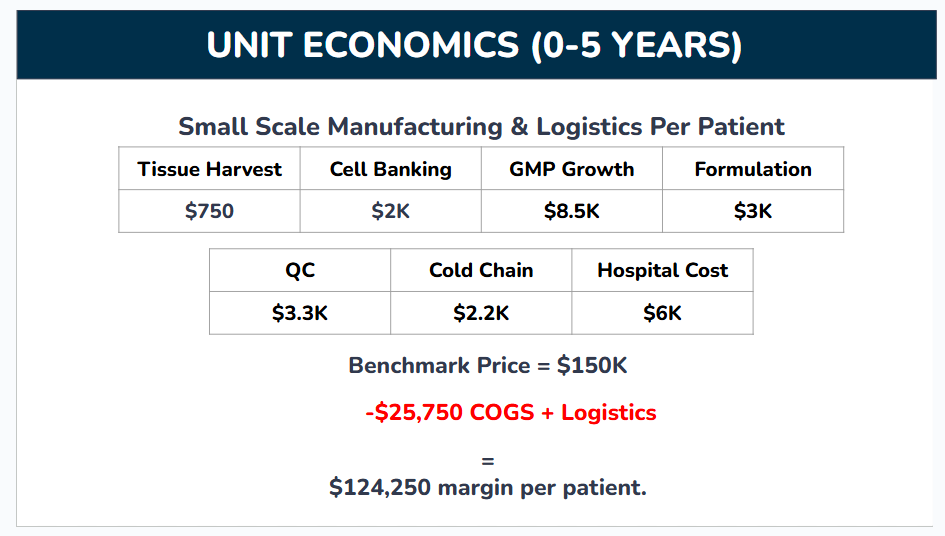
Again, these numbers are rough. Not many companies share their costs publicly, and I had to aggregate data from industry and academia along a range of related cell therapies. Here’s a brief breakdown of each of the items that went into this calculation and how I sourced the data if you’re interested, although you can skip this section without losing sight of the big picture:
Tissue Harvest: The process of getting the feto-maternal material from which our therapies will be created (placental tissue, cord blood, etc). I averaged the cost across a few different tissues for this one, using estimates from nonprofit tissue banks and commercial suppliers.
Cell Banking: Once we extract our brain targeting population from starting tissue, we need to store samples of those cells long term for research, testing, and manufacturing. This was a difficult number to arrive at because so few cost estimates are available and they very broadly between banked cell types and how engineered those cells are. I ended up taking a number from a connection who’s also working on stem cell therapies.
GMP Growth: The population of cells inside our starting tissue that can actually self transport to the brain and exert therapeutic effects is extremely rare. Instead of harvesting large quantities of starting material and mixing cells from different donors into one therapy, we’ll grow the cells we do harvest in the lab until we have enough for multiple therapies. This cell growth needs to happen under GMP (Good Manufacturing Practices) compliant conditions, a stringent set of standards which ensure biological products produced for food or medicine are safe to use. I’m basing this off a review article reporting a cost of $17K to produce 70 million GMP grade mesenchymal stem cells (MSCs). We haven’t locked down the number of cells per full course of our therapy yet, but I don’t expect it’ll surpass 35 million, and took half that cost as our benchmark.
Formulation (or Fill-Finish): The process of turning our cell goop into a deliverable product by packaging it and adding any additives to keep it usable during transport. Again, this is very specific to each individual therapy, and I had to take rough estimates from connections working with stem cells.
Quality Control (QC): All the checks and tests which make sure the medicine is safe for human use. The best source I could find for this was a review article collecting anecdotal costs for different stem cell therapy manufacturers. These costs were reported for a different type of procedure than ours (creating autologous stem cells from patient iPSCs vs harvesting and expanding an allogeneic population from donors), but we can translate their cost/clone as a cost/donor as a rough benchmark. Ignoring the non QC steps reported (reprogramming, etc.) and iPSC specific QC steps (plasmid loss), and after adding a buffer, I estimate about $10K in QC costs per batch. One batch can usually serve many patients, but might only serve 3-4 in the company’s early stages when we’re still struggling to find patients and trials before the batch expires.
Cold Chain: The logistical costs of getting our frozen therapies to patients while maintaining super cold temperatures. Again, needed to estimate from people in the field.
Hospital Cost: The costs incurred by hospital staff as they deliver the therapy. Our therapy is delivered by a very simple IV infusion which can be performed in an outpatient setting (clinics outside of centralized hospitals with less trained staff), and this study reported a cost of $4232 to deliver an infusion of CAR T cells in a similar setting. Adding a bit on top to account for hospital admin fees.
These unit economics are decent for a cell therapy, but they’ll only improve with time. Some costs, such as the tissue harvest, cell banking, and QC, will drop sharply as we serve more patients. Others, such as cold chain logistics and GMP costs, will decline modestly as we benefit from economies of scale. I’ve estimated the unit economics of our therapy after our first five years of operation in the following table:
Total Addressable Market (TAM):
Putting these numbers together with our annual patient estimate, we get a potential global annual market of about $62.8 billion, growing to $66.1 billion after 5 years. This is a respectable revenue potential, surpassing the current $52.8B combined GLP-1 agonist market. And that’s just for ischemic stroke; our vision is to expand these therapies beyond that, creating a platform to repair all brain damage and neurodegenerative disease, and even replace the brain itself.
A Platform for Brain Replacement
I mentioned why we decided to attack ischemic stroke first, and I’m well aware of the saying that a company’s first indication decides their future. However, Sundial Therapeutics isn’t a stroke company. Our vision is not only to bring the first regenerative brain therapy to market and make it accessible off-the-shelf and without surgery, but to build a platform of overlapping technologies allowing us to replace most or all cells in our brains over time, defeating an important aspect of aging by regenerating aged brains with new material and integrating new advances in synthetic biology in order to upgrade human cognition. Every operating system needs updates, even those built on wetware instead of software and hardware. We believe human identity is to be found in the pattern of connections within our brain rather than the physical cells themselves, and aim to preserve those identities over hundreds (or thousands) of years while significantly improving the substrate they run on.
It’s an ambitious mission, one which requires the reality-bending accretions of money and biological expertise hitherto only found in Big Pharma. But we have a plan to get there, and I believe we’ll make it.
Sundial Therapeutics is searching for new collaborators, investors, and team members. If you’re interested in the work we do, please reach out to me at nakoa@sundialthera.bio. And if you’re curious about the science of repairing and replacing brains without surgery, go ahead and: